Case Study of Life Cycle Assessment (LCA) for Chemical Industry
Sodium hydroxide, production of 1 kg mix at a plant
Life Cycle Assessment (LCA) in the Chemical Industry: A case study of the production of 1 kg of Sodium hydroxide at a plant
As well as the kinds and amounts of pollutants emitted into the air, water, and soil, chemicals have distinct environmental profiles determined by their manufacturing process. The environmental implications of chemicals must be studied and analyzed to ensure that they are manufactured and utilized sustainably.
It includes all phases of a chemical’s life cycle, including raw material procurement, product use, and disposal. Life Cycle Assessment (LCA) is a powerful technique for understanding and assessing the environmental consequences of chemicals. It can estimate the impacts of the amount of energy consumed, the type and the effects of the number of pollutants discharged, and the potential for environmental harm overall.
To determine the most sustainable options, LCA can be used to analyze the environmental consequences of various chemicals. Additionally, it may assist with identifying opportunities for reducing environmental impacts, such as enhancing the energy efficiency of chemical manufacturing or switching to more environmentally friendly raw materials.
As well, LCA is a very powerful method of studying and investigating different production chemical synthesis routes. In addition to being challenging to comprehend, LCA results are critical for making educated decisions regarding chemical production and usage from an environmental sustainability standpoint.
How sodium hydroxide can be used
A white substance dissolves in water to form sodium ions (Na+) and hydroxide ions (OH). Sodium hydroxide has the formula NaOH. It is a strong base and alkali. In addition to being used as a catalyst in various chemical processes, sodium hydroxide is used in pulp and paper production, textile production, soap production, and detergent production.
Sodium hydroxide is also used in the food industry as a leavening agent for baking, a pH adjuster for food items, and a regulator for canning products. In addition to using sodium hydroxide as a household cleaner, it can also be used to clean drains, remove oil from kitchen surfaces, and remove hair from sinks and showers. If consumed, sodium hydroxide may be hazardous. It may cause severe skin burns as well as eye impairment.
Caustic soda, also known as lye, is an inorganic chemical with a NaOH formula. It is a white solid ionic compound composed of sodium cations Na+ and hydroxide anions OH. When used at room temperature, sodium hydroxide decomposes proteins and may cause severe chemical burns. It is highly soluble in water and quickly collects moisture and carbon dioxide from the air. crystallizes from water solutions at temperatures 12.3 to 61.8 °C, causing a series of hydrates. It is often used instead of the anhydrous component in commercially available “sodium hydroxide.”
There are several applications for sodium hydroxide.
The aqueous solution of sodium hydroxide is most often used because it is less expensive and easier to handle than aqueous solutions. Sodium hydroxide produces sodium salts and detergents, pH control, and organic synthesis. When a combination needs to be alkalinized or neutralized, sodium hydroxide can be used.
A sodium hydroxide additive is commonly used in drilling mud for increasing alkalinity in bentonite mud systems, increasing viscosity, and neutralizing acid gases that may be encountered during the geological formation (such as hydrogen sulfide and carbon dioxide).
In salt spray tests, where pH must be maintained, sodium hydroxide and hydrochloric acid are used. This salt, NaCl, is the corrosive agent used in conventional neutral pH salt spray tests.
To remove sulfurous contaminants from crude oil, caustic washing uses sodium hydroxide. By interacting with weak acids like hydrogen sulfide and mercaptans, sodium hydroxide produces non-volatile sodium salts that can be removed from the crude oil. In many nations, the procedure is prohibited due to the poisonous and difficult disposal of trash produced. Trafigura used this process in 2006 and disposed of the garbage in Ivory Coast.
Sodium hydroxide is also used in the following applications:
Soap and detergents are made from sodium hydroxide or potassium hydroxide, respectively. For hard bar soap, sodium hydroxide is used, while potassium hydroxide is used for liquid soap.
A drain cleaner that contains sodium hydroxide dissolves fats and grease that might block drains (see cleaning agent).
Rayon, for example, is a synthetic textile fiber.
There is a consumption of sodium hydroxide of around 56% in the paper manufacturing sector, with the paper industry consuming 25% (see chemical pulping).
This is the Bayer process (see dissolving amphoteric metals and compounds) used to purify bauxite ore to recover aluminum metal.
Degreasing metals, refining oil, and making bleaches and colors
Description of the Life Cycle Assessment (LCA) of the case study of the production of 1 kg of Sodium hydroxide at a plant
The Sodium hydroxide, production mix, at plant is evaluated based on the production of 1 kg as a functional unit of The Sodium hydroxide production. The system input is (1) Sodium chloride, (2) Bituminous coal, combusted in industrial boiler, (3) Natural gas, combusted in industrial boiler, (Residual fuel oil, combusted in industrial boiler), and(4) Electricity grid mix (US-based). The full system flow chart of the production is presented in the below figure:
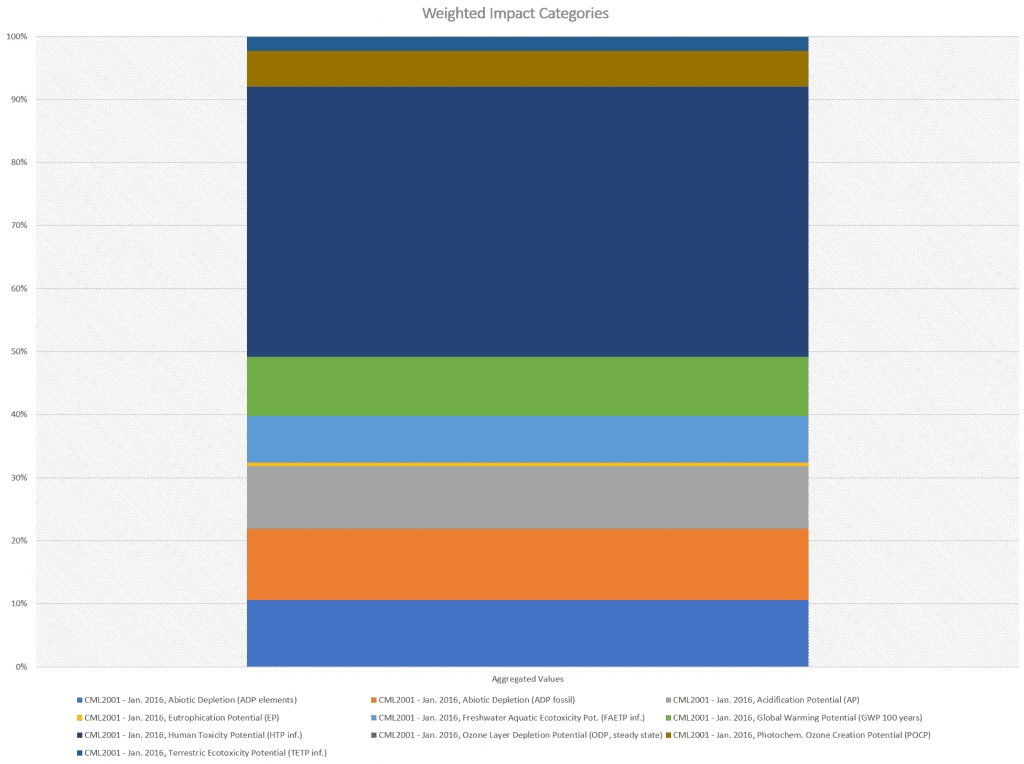
Quantitative environmental impacts in kg equivalent units - System Overall Performance
Results
Abiotic Depletion (ADP elements)
Abiotic Depletion (ADP fossil)
Acidification Potential (AP)
Eutrophication Potential (EP)
Freshwater Aquatic Ecotoxicity Pot. (FAETP inf.)
Global Warming Potential (GWP 100 years)
Human Toxicity Potential (HTP inf.)
Ozone Layer Depletion Potential (ODP, steady state)
Photochem. Ozone Creation Potential (POCP)
Terrestric Ecotoxicity Potential (TETP inf.)
Quantitative environmental impacts in kg equivalent units
Get Started with DEISO LCA Services
Start your project today. Learn about our LCA services or request a quote.